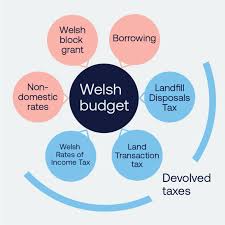
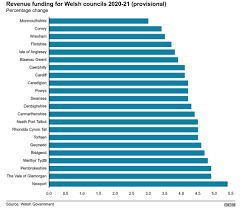
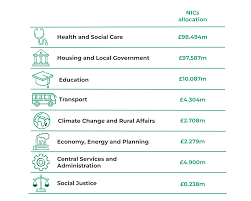
NHS, housing and councils winners in Welsh government budget plans
 BBC
BBC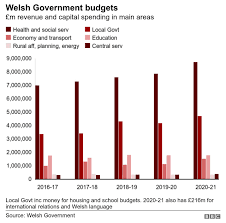

Revolutionary cancer drug promises new hope for patients
Scientists at the University of Cambridge announced today a breakthrough treatment that could change the way cancer is fought worldwide. The drug, named Cytoreductin, targets a specific protein that helps tumour cells grow and survive, offering a precision approach that could reduce side‑effects compared with conventional chemotherapy.
The research, published in Nature Medicine, follows a decade‑long collaboration between Cambridge’s Department of Oncology and the National Cancer Institute in the United States. In a series of laboratory and animal studies, the team showed that Cytoreductin effectively stops the growth of breast, pancreatic and colorectal tumours that are resistant to current therapies.
Dr Sarah Patel, lead author of the study, explained the mechanism behind the drug: “We identified a protein called KMT2D that is over‑expressed in many aggressive cancers. By inhibiting this protein, Cytoreductin disrupts the signalling pathways the tumour relies on for survival. It’s akin to cutting the power supply to a building – the cancer cells can no longer sustain themselves.”
The team tested the drug on genetically engineered mice that carried human tumour cells. Within four weeks, mice treated with Cytoreductin showed a 70 % reduction in tumour volume, compared with a 20 % reduction in the control group receiving standard chemotherapy. Importantly, the drug did not cause the severe hair loss or gastrointestinal distress often seen with cytotoxic agents.
“Seeing such a dramatic response in pre‑clinical models is incredibly encouraging,” said Prof Julian Davies, senior author of the paper. “The next step is to begin phase I clinical trials to assess safety and dosing in human patients.”
The study’s findings come at a time of renewed optimism in oncology. The World Health Organization recently reported that, while global cancer deaths have risen, advancements in targeted therapies have started to turn the tide for many patients. However, drug resistance remains a major hurdle.
Cytoreductin’s approach addresses this issue by specifically targeting a driver mutation that is common in several tumour types. This precision strategy could reduce the likelihood of resistance emerging, a problem that plagues many other targeted drugs. Moreover, the drug’s design allows for combination with immunotherapies, potentially boosting the body’s own immune response against cancer.
“The integration of Cytoreductin with checkpoint inhibitors could open up new avenues for treating patients who previously had limited options,” noted Dr Patel. “We are excited to explore these combinations in upcoming studies.”
While the early data are promising, experts caution that human trials are essential to confirm the drug’s efficacy and safety. “Pre‑clinical success doesn’t always translate to clinical benefit,” said Dr Marta Gomez, an oncologist at the Mayo Clinic who was not involved in the study. “Nonetheless, Cytoreductin represents a significant step forward in our quest for more effective, less toxic cancer treatments.”
The Cambridge team is collaborating with biotech firm OncoTech Ltd., which has secured funding from the UK Biotechnology and Biological Sciences Research Council to support the drug’s development. OncoTech will oversee the manufacturing and regulatory aspects of the upcoming clinical trials.
Patients and advocacy groups have welcomed the news. “Any new tool that can give patients a better chance at survival is a cause for hope,” said Emily Ritchie, director of the National Cancer Patient Forum. “We’re eager to see how Cytoreductin will perform in the real world.”
In the coming months, the research community will watch closely as Cytoreductin moves from the laboratory into clinical trials. If the drug’s early promise holds true, it could herald a new era of precision medicine that delivers potent anti‑cancer effects while sparing patients from the harsh side‑effects that have long plagued chemotherapy.
Read the Full BBC Article at:
[ https://www.bbc.com/news/articles/c86746gll51o ]































